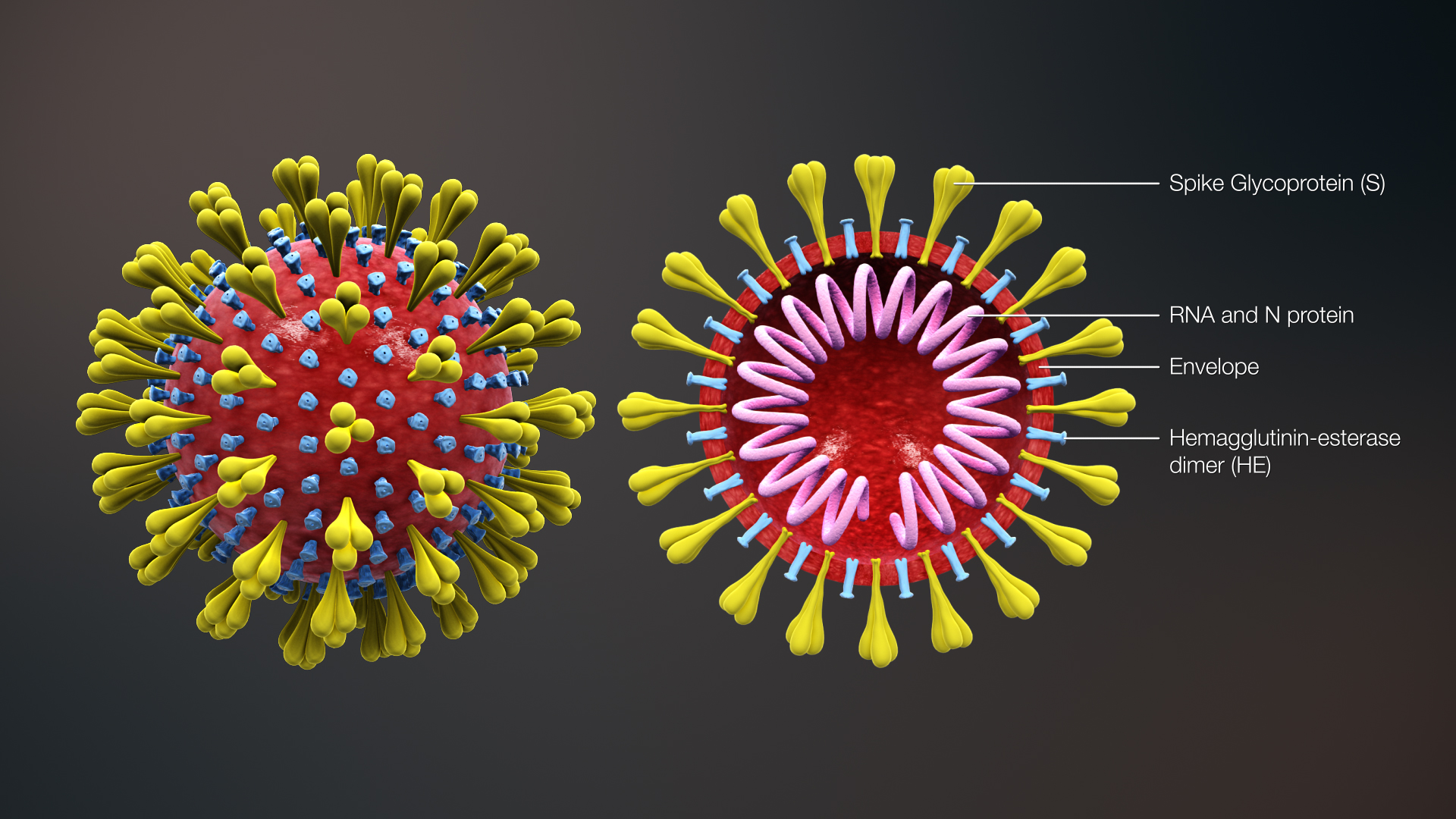
Real-Time RT-PCR Panel for Detection 2019-Novel Coronavirus
- By Southern Labware Marketing
- Feb 28, 2020
Purpose: This document describes the use of real-time RT PCR (rRT-PCR) assays for the in vitro qualitative detection of 2019-Novel Coronavirus (2019-nCoV) in respiratory specimens and sera. The 2019-nCoV primer and probe sets are designed for the universal detection of SARS-like coronaviruses (N3 assay) and for specific detection of 2019-nCoV (N1 and N2 assays).
Protocol Use Limitations: The rRT-PCR assays described here have not been validated for platforms or chemistries other than those described in this document.
Below is a list of necessary equipment, supplies and reagents with links to the products that Southern Labware can provide for you.
Reagents and Supplies
- rRT-PCR primer/probe sets
- Positive template control
- TaqPath™ 1-Step RT-qPCR Master Mix, CG (ThermoFisher; cat # A15299 or A15300)
- Molecular grade water, nuclease-free https://www.southernlabware.
com/molecular-biololgy-grade- water-sterile-depc-treated-1l. html - Disposable powder-free gloves https://www.southernlabware.
com/nitrile-examination- gloves-powder-free-medium- blue-100-pk-1000-cs.html - P2/P10, P200, and P1000 aerosol barrier tips https://www.southernlabware.
com/10ul-filtered-pipette-tip- low-retention-universal-fit- racked-sterile-96-box-10box- case.html - https://www.southernlabware.
com/20ul-filtered-pipette-tip- https://www.southernlabware.low-retention-universal-fit- racked-sterile-96-box-10box- case.html com/200ul-filtered-pipette- tip-low-retention-universal- fit-racked-sterile-96-box- 10box-case.html - Sterile, nuclease-free 1.5 mL microcentrifuge tubes https://www.southernlabware.
com/sureseal-stm-1-5ml- microcentrifuge-tube-sterile- packed-in-bags-of-50- microtubes-10-bags-of-50-case. html - 0.2 mL PCR reaction tube strips or 96-well real-time PCR reaction plates and optical 8-cap strips https://www.southernlabware.
com/pure-amptm-pcr-tubes- strips-0-2ml-qpcr-8-strip- with-separate-optical-strip- caps-natural-clear-120-pkg. html - Laboratory marking pen
- Cooler racks for 1.5 microcentrifuge tubes and 96-well 0.2 mL PCR reaction tubes https://www.southernlabware.
com/coolcubetm-pcr- https://www.southernlabware.workstation.html com/pcrr-cooler-96-well-light- blue-2-ea.html - Racks for 1.5 ml microcentrifuge tubes https://www.southernlabware.
com/80-well-micro-tube-racks- assorted-5-pacl.html - Acceptable surface decontaminants
- DNAZapTM (Life Technologies, cat. #AM9890)
- DNA AwayTM (Fisher Scientific; cat. #21-236-28)
- RNAse AwayTM (Fisher Scientific; cat. #21-236-21
- 10% bleach (1:10 dilution of commercial 5.25-6.0% sodium hypochlorite)
Equipment
- PCR Work Station [UV lamp; Laminar flow (Class 100 HEPA filtered)] https://www.southernlabware.
com/mystairer-my-pcr-24- https://www.southernlabware.cleanprep-pcr-workstation- circulation-free-enclosure- with-timed-uv-light-iso-5- clean-110v.html com/mystairer-my-pcr-32- https://www.cleanprep-pcr-workstation- circulation-free-enclosure- with-timed-uv-light-iso-5- clean-110v.html southernlabware.com/mystairer- my-pcr-48-cleanprep-pcr- workstation-circulation-free- enclosure-with-timed-uv-light- iso-5-clean-110v.html - Vortex mixer https://www.southernlabware.
com/mini-vortex-mixer.html - Microcentrifuge https://www.southernlabware.
com/laboratory-equipment/ centrifuges/oxford-benchmate- micro-centrifuge-8-place-x-1- 5-2-0ml-capacity-with-pcr- strip-rotor-6000rpm-2000xg- fixed-speed.html - Micropipettes (2 or 10 µl, 200 µl and 1000 µl) https://www.southernlabware.
com/propette-pipettor-0-1-to- https://www.southernlabware.2ul-9077.html com/propette-single-channel- https://www.southernlabware.pipettor-variable-volume-20- to-200ul.html com/propette-single-channel- pipettor-variable-volume-100- to-1000ul.html - Multichannel micropipettes (5-50 µl) https://www.southernlabware.
com/propettetm-le-multi- channel-pipettors-8-channel-5- 0-to-50ul-8774.html - 2 x 96-well cold blocks https://www.southernlabware.
com/coolcubetm-pcr- https://www.southernlabware.workstation.html com/pcrr-cooler-96-well-light- blue-2-ea.html - -20oC (nonfrost-free) and -70oC freezers; 4oC refrigerator https://www.southernlabware.
com/arctiko-under-counter- uluf-15-ultra-low-freezer-0-3- cu-ft-40-86-c-110v.html - Real-time PCR detection system
- Nucleic acid extraction system